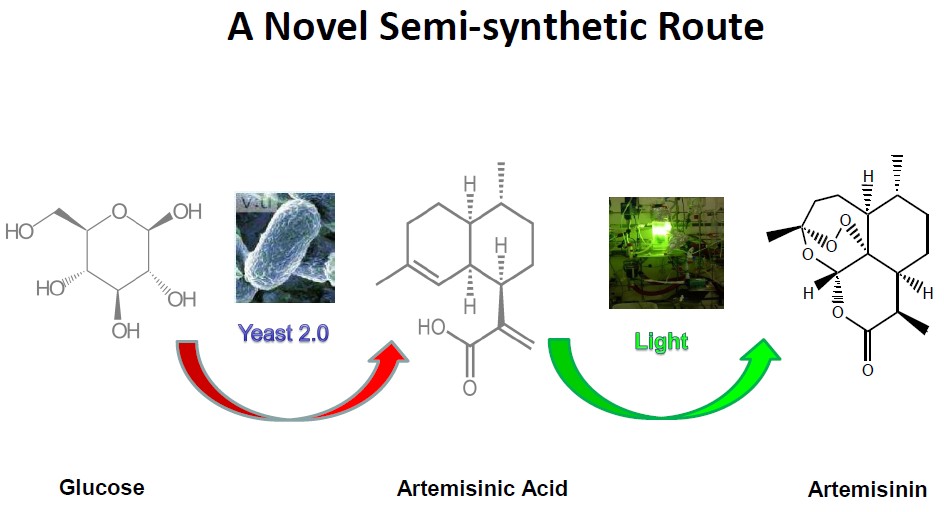
Artemisinic acid is vital to making artemisinin for anti-malaria treatment. Artemisinic acid is isolated from the leaves by extraction (continuous hot percolation) in non polar solvents (usually n-hexane) and separation from the extract, using n-hexane and aqueous acetonitrile. Purification treatments include partial crystallisation of artemisinic acid from the acetonitrile phase and column chromatography of the residue using ethyl acetate/hexane mixtures leading first to artemisinic acid and then to artemisinin in the following fractions. Reduction of the exomethylen group of artemisinic acid into DHAA is carried out using LiBH4 or NaBH4 in the presence of nickel chloride (or its hexahydrate). DHAA is converted into artemisinin through photo-oxidation by oxygen or air and irradiation with a visible light source, in the presence of a photosensitizer, or photo-oxidation via oxidation and irradiation in the presence of fluorescent light. DHAA can also be converted into artemisinin via non-photochemical oxidation in the presence of hydrogen peroxide and a metal catalyst.BSgShanghai Natural Bio-engineering Co., Ltd
| product name |
Artemisinic acid |
| Source |
leaf of Artemisia annua L |
| Synonyms |
artemisic acid, CHEBI:63749, 80286-58-4, SureCN159983, CHEMBL457385, UNII-53N99527G7, W2206, C20309, 222F86B0-6F56-4D85-98B2-F1B0E146A747 |
| Specification |
95%-99% |
| Molecular Formula |
C15H22O2 |
| Molecular Weight |
234.33398 |
| CAS |
80286-58-4 |
Artemisinic acid, isolated as the active principles of the medicinal plant Artemisia annua. Artemisinic acid, is a major precursor of artemisinin (an antimalaric compound), isolated as the active principles of the medicinal plant Artemisia annua L. Malaria remains a major global health problem, killing about one million people each year, with the protozoan parasite Plasmodium falciparum responsible for this most deadly illness. The sesquiterpene endoperoxide artemisinin is currently the most effective treatment against multi-drug resistant Plasmodium species, and artemisinin combination treatments (ACTs) are now first-line drugs, as recommended by the WHO. The compound is extracted from the plant Artemisia annua (sweet wormwood), now cultivated in many countries for this purpose. However, reliance on cultivated A. annua restricts the supply of the drug and elevates costs for the patients. The supply of plant-derived artemisinin is unstable, resulting in shortages and price fluctuations, complicating production planning by ACT manufacturers. A stable source of affordable artemisinin is required.
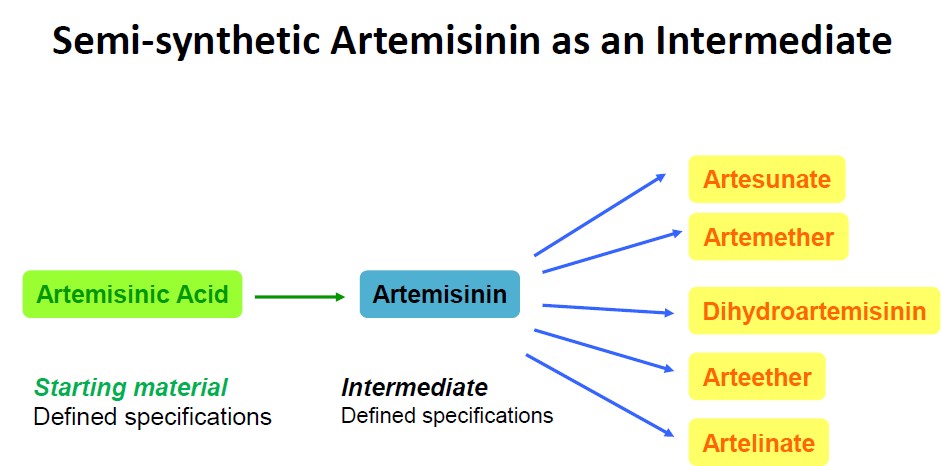
The total synthesis of artemisinin is too laborious to be considered a viable alternative for supplying the highly costsensitive market. Although total synthesis of artemisinin is difficult and costly, the semi-synthesis of artemisinin or any derivative from microbially sourced artemisinic acid, its immediate precursor, could be a cost-effective, environmentally friendly, high-quality and reliable source of artemisinin.
Artemisinic acid, a much-less complex molecular precursor, can be extracted from the same plant in higher yields, or produced in engineered yeast. Therefore, artemisinic acid is an ideal starting point for synthetically producing artemisinin. Still, the conversion of artemisinic acid to artemisinin has proven a formidable challenge for chemists, since a high-yielding, scalable, and low-cost process for constructing a highly complex molecule is needed.
A group of researchers made semi-synthetic artemisinin possible after they used synthetic biology to develop strains of the yeast Saccharomyces cerevisiae for high-yielding biological production of artemisinic acid, a precursor of artemisinin.
 BSgShanghai Natural Bio-engineering Co., Ltd
BSgShanghai Natural Bio-engineering Co., Ltd
Preparation of artemisinin from artemisinic acid
Artemisia annua contains far more artemisinic acid than artemisinin. A concern has been improving the yield of extracted artemisinin via extraction of artemisinic acid from Artemisia annua leaves and conversion into artemisinin. This route to artemisinin comprises reduction to dihydroartemisinic acid (DHAA) with alcaline borohydride and photo-oxidation of DHAA followed by air-oxydation until complete formation of artemisinin, or non-photochemical oxydation.
Hunan keyuan Bio-products co., Ltd is the manufacturer of artemisinic acid and artemsinin for anti-malaria treatment.
If you have inquiry, please kindly let us know.
BSgShanghai Natural Bio-engineering Co., Ltd
Share to:
This article addresses:http://www.hnkeyuan.com/news/Industry News/Artemisinic-acid-artemisinin.html
Next:Synthetic Biology's Malaria Promises Could Backfire
Previous: How to distinguish natural resveratrol from synthetic resveratrol?